a4
KRCEEAdmin2021-02-04T15:26:05-05:00Why Enrichment?
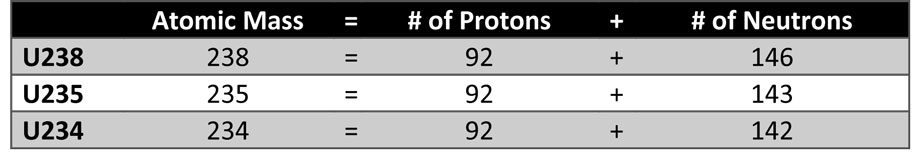
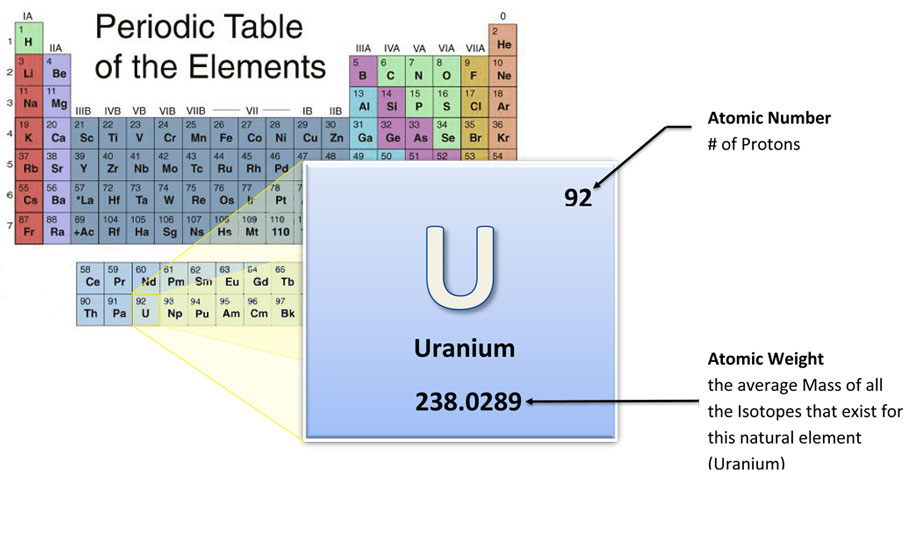
Uranium cannot be used as a fuel or weapon in its natural state. To be used as reactor fuel or bomb manufacture, U-235 must be separated from natural uranium.
U-235 is the only fissile isotope in natural Uranium (U), therefore Uranium must be “enriched” to have a higher percent concentration of U-235.